We are investigating the potential of reprogramming Müller glia into neurons in the mouse, non-human primate and human retina. To this end, we have taken lessons from naturally regenerating species, such as the zebrafish, which overexpress proneural transcription factors in response to injury to replenish lost neurons. We have successfully found a way to regenerate both amacrine cells and bipolar cells, two types of retinal interneurons, by over-expressing the proneural transcription factor Ascl1, or the combinations Ascl1-Atoh1, Ascl1-Islet1-Pou4f2. The newborn neurons integrate with the existing circuitry and respond to light stimuli. Importantly, we can stimulate neurogenesis after the loss of both retinal ganglion cells or the loss of photoreceptors in adult mice.
reprogramming Müller glia
We can reinstate some regenerative capacity in mammalian glia by overexpressing proneural genes.
adult neurogenesis
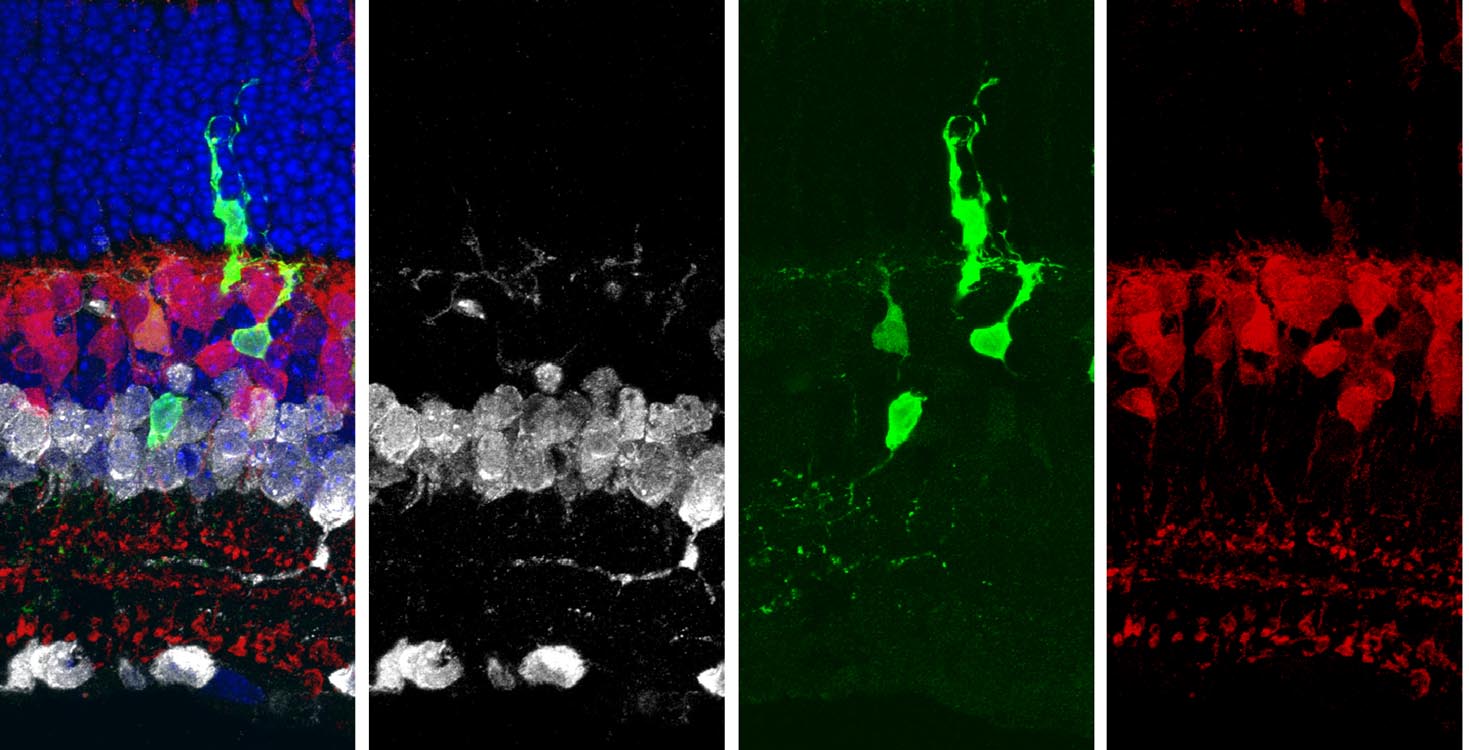
Overexpression of Ascl1 in adult Müller glia after ablating photoreceptors stimulates neurogenesis (arrows).
Though most of our foundational work was using transgenic animal models, we have now transitioned to the use of viral vector strategies to drive proneural genes in the adult retina with the aim of establishing a regenerative therapy. We show that vector-mediated reprogramming phenocopies the pattern of neurogenesis we obtain when driving proneural genes transgenically.
Vector-mediated expression of Ascl1 in Müller glia drives neurogenesis of Otx2+ bipolar neurons in adult mice.